Physical Experience
 Water is unique. While its physical and chemical properties are common and simple, they are often exceptions. For example, at 4°C, the density of water is maximal, and in the transition to solid state (old) it is reduced! No other substance does.
Water is unique. While its physical and chemical properties are common and simple, they are often exceptions. For example, at 4°C, the density of water is maximal, and in the transition to solid state (old) it is reduced! No other substance does.
As for this experience, it seems clear and simple. The water is washing paper and bleach, and the materials are wet. Well, explaining why this is happening, it's hard.
We'll deal, for starters, with the term " laughing " . It is a phenomenon of fluid interaction with a solid body surface. Development options are, as always, two:
- The tension between molecules of liquid is stronger than their attraction to solid molecules. Liquidity seeks to reduce contact with the surface and, as a result, meets in drops.
- The tension between molecules of liquid is weaker than their attraction to solid molecules. Liquidity seeks to increase the contact area and, as a result, clutch to the body ' s surface through it.
There's obviously a second option. The flow occurs until the fluid covers the whole surface or until the layer of liquid becomes monomolecular.
But how does water overcome gravity?
As in the plants. The water rises upstream of the plant ' s drip receptacles and delivers it from roots to leaves and fruit.
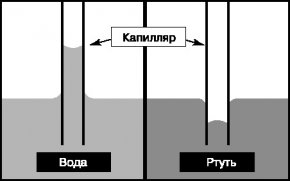
This occurs through the differential pressures and surface tension forces. The surface of the water entering a narrow capillare takes the interior shape (menice). Under this condition, the fluid pressure under this menian becomes less atmospheric and the water is upwards. And the thicker the drip, the higher the water rises to balancing the negative pressure. If the liquid doesn't smash the surface, the meniscus will be scared and it won't rise up the hood.
The shelf has a porous structure and consists mainly of pulp, which in turn has a fibrous structure. Thus, it is not difficult for water to find paths for upstream traffic.






