Simple Chemical Home Experiences
 Chemical experience with powder: how the powder explodes!
Chemical experience with powder: how the powder explodes!
Porn
Smoke, or black, powder is a mixture of potassium selitis (Calium nitrate - KNO3), sulphur (S) and coal (C). It's flammable at about 300 °C. The powder could be blown up. It consists of oxidant (seliter) and reinstater (coal). The serial is also a reinstater, but its primary function is to connect the calium into a solid compound. When the powder burns, there's a reaction:
2KNO3+C+S circle K2S+N2+3CO2,
- which gives a large volume of gaseous substances. The use of gunpowder in a military case involves the use of gunpowder: the gases produced by the explosion and the heat-emitting reactions push the bullet out of the weapon. In the formation of potassium sulphide, it's easy to see by smelling the gun. It smells hydrogen sulphide, a hydrolysis potassium sulphide product.
 Chemical experience with selium:
Chemical experience with selium:
Effective chemical experience You can do it with a saliva. I'll remind you that sélitres are complex substances - salts of nitrogen acid. In this case, we're gonna need a potassium saliva. Her chemical formula KNO3. On the paper sheet, paint the contour, the drawing (for more effect, let the lines not intersect!) Prepare a concentrated potassium nitrate solution. For information: 20 g KNO3 is dissolved in 15 ml.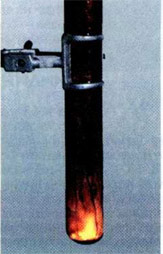 Then, by the brush, we'll recharge the paper on the painted contour, without leaving the passes and wires, and we'll dry the paper. Now we need to touch a burning radius of some point on the contour. The same time there will be a scream, which will move slowly on the end of the drawing until it is completely closed. That's happens what: The calium selita is degraded by equation:
Then, by the brush, we'll recharge the paper on the painted contour, without leaving the passes and wires, and we'll dry the paper. Now we need to touch a burning radius of some point on the contour. The same time there will be a scream, which will move slowly on the end of the drawing until it is completely closed. That's happens what: The calium selita is degraded by equation:
2KNO3 circle 2 KNO2 +O2.
Here KNO2 +O2 is the salt of nitrogenic acid. Oxygen-designed paper is glued and burned. For greater impact, experience can be conducted in a dark room.
Chemical experience of glazing in floating acid
Glass dissolved
in floating acid










